Technical Note Number 4
NITRITES AND
THEIR IMPACT
ON EFFLUENT CHLORINATION
Chlorination of secondary treatment effluents is some times impaired by an
immediate chlorine demand exerted by nitrites. Such a demand is erratic and can
be so large as to prevent maintaining a chlorine residual regardless of the
dosage. In most instances, the condition appears to be transitory and soon
disappears. However, in some instances, especially in the case of aerated
lagoons with long retention times, the condition lasts long enough to result in
fecal coliform violations of the effluent discharge permit. Remedial measures to
remedy the problem depends primarily on the understanding of the conditions that
favor nitrite production.
Cool-Water Accumulation of Nitrites
Nitrification (oxidation of ammonia to nitrate) is a two step process.
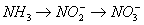 (1)
(1)
Under aerobic conditions, with sufficient alkalinity, and a
favorable temperature, ammonia (NH3) is oxidized to nitrite (NO2-)
which in turn, is oxidized to nitrate (NO3-). At
temperatures above approximately 17°C,
the first step, the oxidation of ammonia to nitrite, is the slowest step.
Consequently, when nitrite is formed, it is rapidly oxidized to nitrate,
resulting in a relatively low ambient concentration of nitrite (< 1-2 mg/L)
being found in the effluent. At temperatures below 17°C,
the rate of nitrite oxidation to nitrate begins to decrease until, at a
temperature of from 12°-14°C,
the rate of nitrite oxidation to nitrate becomes the rate controlling step in
nitrification. Under such temperature conditions, significant nitrite
accumulation can occur (>15 mg/L) (Randall and Buth 1984). In addition, there
can be specific compounds introduced by industrial discharges that exert a
differential toxicity on the organisms responsible for the two steps, thereby
resulting in nitrite accumulation.
About
the only remedy that can be suggested for cold water nitrite formation is to
limit the nitrification process by reducing the level of aeration. Usually,
accumulations of nitrite that occurs in the spring are transitory and will
disappear with warmer weather.
Accumulations that occurs in the fall will dissipate and disappear as
nitrification ceases with falling temperature.
Warm Water Accumulation of Nitrites
Nitrite
accumulation can also occur under conditions in which temperatures are above 17°C.
When oxygen is limiting in parts of the aerated lagoon system, any nitrates that
have been produced in other parts of the system that have been aerobic will
become reduced by denitrification. Denitrification can be simplified as a
two-step process.
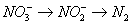 (2)
(2)
However, unlike in nitrification, the second
step appears to be the slowest step (Dawson and Murphy 1972), particularly if
the carbon is limiting growth (Freedman 1999) or if the carbon source is complex
(McCarty et al. 1969). Furthermore, it has been found that the second
step, nitrite reduction, is inhibited by the presence of nitrates (Kornaros
et al. 1996), and is more sensitive to oxygen (Kornaros and Lyberatos 1998).
Since the second step is the slowest step, nitrite can accumulate in significant
concentrations.
When the condition of excessive nitrites in the effluent
of an aerated lagoon operating at a temperature >17°C
persists, the best remedy is to attempt to nitrify the nitrites to nitrates by
ensuring a completely aerobic environment (all aerators on all the time) along
with at least an effluent alkalinity of 150 mg/L.
Impact of Ammonia Addition
on Chlorine Demand
When
chlorine is added to effluents with nitrites but with little ammonium nitrogen,
the chlorine reacts as free chlorine and is removed quickly by a chemical
reaction with the nitrites, thereby increasing significantly the amount of
chlorine that must be used to meet the required limit of coliform concentration.
Each mg/L of nitrite nitrogen reacts with 5 mg/L of chlorine. Thus, a nitrite
concentration of only 10 mg/L will exert a chlorine demand of about 50 mg/L.
It has been shown, however, that when chlorine is added to effluents
with nitrites and with a high concentration of ammonium ion (>20 mg/L), the
chlorine reacts preferentially with the ammonium ion forming chloramines
(Phoenix 1995). This suggests that, if insufficient ammonium is already present
in the effluent, the chlorine demand exerted by effluent nitrites can be
minimized by adding ammonia along with chlorine in a well-mixed chlorine contact
basin. Chloramines, while effective as disinfectants, will act only slowly with
nitrites (Chen and Jenson 2001).
REFERENCES
Chen, W. L. and Jensen, J.
N. (2001). “Effect of chlorine demand on the breakpoint curve: Model
development, validation with nitrite, and application to municipal wastewater.”
Water Environ. Res., 73(6), 721.
Dawson, P. L. and Murphy, K.
L. (1972). “The temperature dependence of biological denitrification.” Proc.
24th Industrial Waste Conf., Purdue Univ., Lafayette, IN.
Freedman, D. L. (1999).
Personal communication. Clemson Univ., Clemson, SC.
Kornaros, M. et al.
(1996). “Kinetics of denitrification by Pseudomonas denitrificans under
growth conditions limited by carbon and/or nitrate or nitrite.” Water Envir.
Res., 68(5), 934-945.
Kornaros, M. and Lyberatos,
G. (1998). “Kinetic modeling of Pseudomonas denitrificans growth and
denitrification under aerobic, anoxic, and transient operating conditions.”
Wat. Res., 32(6), 1912-1922.
McCarty, P. L. et al.
(1969). “Biological denitrification of wastewaters by the addition of organic
materials. ”Water Res., 6, 71-83.
Phoenix, City of (1995).
Water Services Department Task Report 2010.
Randall, C. W. and Buth,
David (1984). “Nitrite buildup in activated sludge resulting from temperature
effects.” J. Water Pollut. Control Fed., 56(9),1039.

